Zinc Acetate Dihydrate Anhydrous USP BP Ph Eur Analytical Reagent Grade & Zinc Citrate Dihydrate Anhydrous USP Grade Suppliers Exporters, Manufacturers
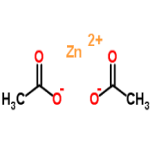
Zinc Acetate
CAS Number: 5970-45-6 Dihydrate 557-34-6 Anhydrous USP BP Ph Eur Analytical Reagent Grade Suppliers Exporters, Manufacturers

Please visit Safety Data Sheet of Zinc Acetate Dihydrate Anhydrous USP BP Ph Eur Analytical Reagent Grade Manufacturers.
Zinc Acetate USP Grade Specifications:
C4H6O4Zn-2H2O --- 219.50
C4H6O4Zn --- 183.48
Acetic acid, zinc salt, dihydrate;
Zinc acetate dihydrate CAS 5970-45-6
Zinc acetate anhydrous CAS 557-34-6
DEFINITION
Zinc Acetate contains NLT 98.0% and NMT 102.0% of C4H6O4Zn-2H2O.
IDENTIFICATION
A. Identification Tests - General, Zinc: A 50 mg/mL solution meets the requirements.
B. Identification Tests - General, Acetate: A 50 mg/mL solution meets the requirements.
ASSAY
Procedure
Sample solution: To 400 mg of Zinc Acetate in 100 mL of water add 5 mL of ammonia-ammonium chloride buffer and 0.1 mL of eriochrome black.
Analysis: Titrate the Sample solution with 0.05 M edetate disodium until the solution is deep blue in color. Each mL of 0.05 M edetate disodium is equivalent to 10.98 mg of zinc acetate dihydrate (C4H6O4Zn-2H2O).
Acceptance criteria: 98% to 102%
Chloride and Sulfate, Sulfate: A 1.0-g portion shows no more sulfate than corresponds to 0.10 mL of 0.020 N sulfuric acid (0.010%).
Arsenic: NMT 3 ppm
Lead: To pass the test, --- (0.002%).
Alkalies and Alkaline Earths:
Sample solution: Dissolve 2.0 g in 150 mL of water in a 200-mL volumetric flask. Add sufficient ammonium sulfide TS to precipitate the zinc completely and dilute with water to volume. Pass through a dry filter, rejecting the first portion of the filtrate.
Analysis: To 100 mL of the filtrate add 5 drops of sulfuric acid. Evaporate to dryness and ignite.
Acceptance criteria: The weight of the residue does not exceed 2 mg (0.2%).
Insoluble Matter: A 20-g portion, dissolved in 150 mL of water containing 1 mL of glacial acetic acid, shows NMT 1.0 mg of insoluble matter (0.005%).
pH: 6 to 8, in a solution (1 in 20)
Packaging and Storage: Preserve in tight containers.
Specifications of Zinc Acetate BP Ph Eur Grade:
C4H6O4Zn-2H2O --- 219.5 --- CAS 5970-45-6
Action and use: Astringent.
DEFINITION
Content: 99.0 per cent to 101.0 per cent of C4H6O4Zn,2H2O.
CHARACTERS
Appearance: White or almost white crystalline powder or flakes.
Solubility: Freely soluble in water, soluble in ethanol (96 per cent).
IDENTIFICATION
A. It gives reaction of acetates.
B. It gives the reaction of zinc.
TESTS
Solution S: Dissolve 10.0 g in carbon dioxide-free water prepared from distilled water and dilute to 100 mL with the same solvent.
Appearance of solution: Solution S is clear and colourless.
pH: 5.8 to 7.0.
Dilute 10 mL of solution S to 20 mL with carbon dioxide-free water.
Reducing substances: Boil for 5 min a mixture of 10 mL of solution S, 90 mL of water, 5 mL of dilute sulfuric acid and 1.5 mL of a 0.3 g/L solution of potassium permanganate. The pink colour of the solution remains.
Chlorides: Maximum 50 ppm.
Sulfates: Maximum 100 ppm, determined on solution S.
Aluminium: Maximum 5 ppm.
Arsenic: Maximum 2 ppm, determined on 0.5 g.
Cadmium: Maximum 2 ppm.
Copper: Maximum 50 ppm.
Iron: Maximum 50 ppm.
Lead: Maximum 10 ppm.
Specifications of Zinc Acetate Dihydrate Reagent
(CH3COO)2Zn-2H2O
Formula Weight 219.51
CAS Number 5970-45-6
REQUIREMENTS
Assay: 98.0-101.0% (CH3COO)2Zn-2H2O
pH of a 5% solution: 6.0-7.0 at 25C
MAXIMUM ALLOWABLE
Insoluble matter: 0.005%
Chloride (Cl): 5 ppm
Sulfate (SO4): 0.005%
Calcium (Ca): 0.005%
Magnesium (Mg): 0.005%
Potassium (K): 0.01%
Sodium (Na): 0.05%
Iron (Fe): 5 ppm
Lead (Pb): 0.002%.
Please visit Hazard Statement of Zinc Acetate Dihydrate Anhydrous USP BP Ph Eur Analytical Reagent Grade Suppliers.
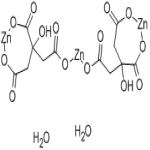
Zinc Citrate
CAS Number: 546-46-3 Anhydrous & 5990-32-9 Dihydrate Dihydrate Anhydrous USP Grade Suppliers Exporters, Manufacturers

Please visit Safety Data Sheet of Zinc Citrate Dihydrate Anhydrous USP Grade Manufacturers.
Zinc Citrate Dietary Supplement USP Grade:
C12H10O14Zn3-2H2O --- 610.36
2-Hydroxy-1,2,3-propanetricarboxylic acid zinc salt, dihydrate CAS 5990-32-9
Anhydrous CAS 546-46-3
DEFINITION
Zinc Citrate contains NLT 31.3% of zinc (Zn), calculated on the dried basis.
IDENTIFICATION
A. Identification Tests - General, Zinc: A solution (1 in 10) meets the requirements.
B. ;Identification Tests - General, Citrate: A solution (1 in 10) meets the requirements.
ASSAY
Procedure
Sample: 350 mg of Zinc Citrate, previously dried at 105C for 2 h
Blank: 60 mL of water
Titrimetric system
Mode: Direct titration
Titrant: 0.05 M edetate disodium
Endpoint detection: Visual
Analysis: Dissolve the Sample in 60 mL of water. Add 10 mL of ammonia-ammonium chloride buffer and 0.1 mL of eriochrome black. Titrate with the Titrant to a blue endpoint. Perform a blank determination.
Calculate the percentage of & zinc (Zn) in the portion of Zinc Citrate taken:
Result = [(V − B) x M x F x 100]/W
V = sample titrant volume (mL)
B = blank titrant volume (mL)
M = titrant molarity (mM/mL)
F = equivalency factor, 65.4 mg/mM
W = sample weight (mg)
Acceptance criteria: NLT 31.3% on the dried basis.
Chloride and Sulfate, Chloride: A 1.0-g portion shows no more chloride than corresponds to 0.7 mL of 0.020 N hydrochloric acid (NMT 0.05%).
Chloride and Sulfate, Sulfate: A 1.8-g portion shows no more sulfate than corresponds to 0.5 mL of 0.020 N sulfuric acid (NMT 0.05%).
Limit of Arsenic, Cadmium, and Lead: To pass the test.
Arsenic: NMT 3 µg/g
Cadmium: NMT 5 µg/g
Lead: NMT 10 µg/g
Loss on Drying: Dry a sample at 105C for 2 h: it loses NMT 1.0% of its weight.
Microbial Enumeration Tests: The total aerobic microbial count does not exceed 103 cfu/g. The total combined yeasts and molds count does not exceed 102 cfu/g.
Microbiological Procedures for Absence of Specified Microorganisms: It meets the requirements of the test for absence of & Escherichia coli.
Please visit Hazard Statement of Zinc Citrate Dihydrate Anhydrous USP Grade Suppliers.
Zinc Acetate CAS Number 5970-45-6 Dihydrate 557-34-6 Anhydrous & Zinc Citrate Dihydrate Anhydrous CAS Number 546-46-3 Anhydrous & 5990-32-9 Dihydrate Supplier Exporter, Manufacturer:
Annie Chemie P Ltd
Mumbai 4000010, INDIA
With Agents and offices in UAE, USA, Europe.
e-mail: info@anniechemie.com
Copyright and Usual Disclaimer is Applicable.
June 3, 2025
Exporters to USA, Canada, UK, Europe, UAE, Nigeria, Algeria, Turkey, Mexico, Brazil, Chile, Argentina, Australia, Dubai etc.
Perfection is made up of small things and that is a big thing.
