Hyaluronic Acid & Sodium Hyaluronate USP NF BP Ph Eur Grade Suppliers Exporters, Manufacturers
Hyaluronic Acid
CAS Number: 9004-61-9, Suppliers Exporters, Manufacturers
Please visit Safety Data Sheet of Hyaluronic Acid Manufacturers.
Specifications of Hyaluronic Acid:
Appeararice: White powder
Mesh I00% pass through: All passes 80 mesh
Hyaluronic Acid: 95.0 % 99%
Molecular weight (1.0-1.5) x 1000000
Glucuronic acid: 45.0% to 47%
Loss on drying: Maximum 10%
pH(0.1%Solution): 6.0-7.5
Transparency (0.1 % solution): >99. to 99.5%
Proteins (drv basis): Maximum 0.l%
Residue on ignition: Maximum 20%
Heavy Metals: Maximum 20ppm
Arsenic: Maximum 2ppm
Total Plate Count: Maximum 100cfu/gm
Yeast and molds: Maximum 10cfu/gm
Staphylococcus aureus: Negative
Pseudomonas aeruqinosa: Negative.

Please visit Hazard Statement of Hyaluronic Acid Manufacturers.
Sodium Hyaluronate
CAS Number: 9067-32-7, USP NF BP Ph Eur Grade Suppliers Exporters, Manufacturers

Please visit Safety Data Sheet of Sodium Hyaluronate Manufacturers.
Specifications of Sodium Hyaluronate BP Ph Eur Grade
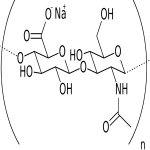
(C14H20NNaO11)n --- CAS 9067-32-7
Action and use: High viscosity mucopolysaccharide.
DEFINITION
Sodium salt of hyaluronic acid, a glycosaminoglycan consisting of D-glucuronic acid and Nacetyl- D-glucosamine disaccharide units.
Content: 95.0 per cent to 105.0 per cent (dried substance).
Intrinsic viscosity: 90 per cent to 120 per cent of the value stated on the label.
PRODUCTION
It is extracted from cocks' combs or obtained by fermentation from Streptococci, Lancefield Groups A and C. When produced by fermentation of gram-positive bacteria, the process must be shown to reduce or eliminate pyrogenic or inflammatory components of the cell wall.
CHARACTERS
Appearance: White or almost white, very hygroscopic powder or fibrous aggregate.
Solubility: Sparingly soluble or soluble in water, practically insoluble in acetone and in anhydrous ethanol.
IDENTIFICATION
A. Infrared absorption spectrophotometry. ComparisoniPh. Eur. reference spectrum of sodium hyaluronate.
B. It gives reaction of sodium.
TESTS
Solution S: Weigh a quantity of the substance to be examined equivalent to 0.10 g of the dried substance and add 30.0 ml of a 9 g/l solution of sodium chloride. Mix gently on a shaker until dissolved (about 12 h).
Appearance of solution: Solution S is clear and its absorbance at 600 nm is not greater than 0.01.
pH: 5.0 to 8.5.
Dissolve the substance to be examined in carbon dioxide-free water to obtain a solution containing a quantity equivalent to 5 mg of the dried substance per millilitre.
Intrinsic viscosity: To pass the test.
Sulphated glycosaminoglycans: Maximum 1 per cent, if the product is extracted from cocks' combs.
Nucleic acids: The absorbance of solution S at 260 nm is maximum 0.5.
Protein: Maximum 0.3 per cent; maximum 0.1 per cent, if intended for use in the manufacture of parenteral preparations.
Chlorides: Maximum 0.5 per cent.
Iron: Maximum 80.0 ppm.
Heavy metals: Maximum 20 ppm; maximum 10 ppm if intended for use in the manufacture of parenteral preparations.
Loss on drying: Maximum 20.0 per cent, determined on 0.500 g by drying at 100-110C over diphosphorus pentoxide for 6 h.
Microbial contamination: Total viable aerobic count not more than 100 micro-organisms per gram. Use 1 g of the substance to be examined.
Bacterial endotoxins: Less than 0.5 IU/mg, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins; less than 0.05 IU/mg, if intended for use in the manufacture of intra-ocular preparations or intra-articular preparations without a further appropriate procedure for the removal of bacterial endotoxins.
Sodium Hyaluronate USP NF Grade can also be offered.
Please visit Hazard Statement of Sodium Hyaluronate USP NF BP Ph Eur Grade Manufacturers.
Hyaluronic Acid CAS Number 9004-61-9 & Sodium Hyaluronate USP NF BP Ph Eur Grade CAS Number 9067-32-7 Supplier Exporter, Manufacturer:
Annie Chemie P Ltd
Mumbai 4000010, INDIA
With Agents and offices in UAE, USA, Europe.
e-mail: info@anniechemie.com
Copyright and Usual Disclaimer is Applicable.
May 31, 2025
Exporters to USA, Canada, UK, Europe, UAE, Nigeria, Algeria, Turkey, Mexico, Brazil, Chile, Argentina, Australia, Dubai etc.
Perfection is made up of small things and that is a big thing.
