Fumaric Acid USP NF FCC Food Grade & Ferrous Fumarate USP BP Ph Eur IP FCC Food Grade Suppliers Exporters, Manufacturers
Fumaric Acid
CAS Number: 110-17-8, USP NF FCC Food Grade Suppliers Exporters, Manufacturers
Please visit Safety Data Sheet of Fumaric Acid Manufacturers.
Specifications of Fumaric Acid USP NF
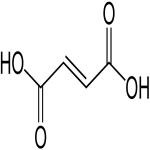
C4H4O4 --- 116.07
2-Butenedioic acid, E-.
Fumaric acid CAS 110-17-8
Fumaric Acid contains not less than 99.5 percent and not more than 100.5 percent of C4H4O4, calculated on the anhydrous basis.
Identification: Dissolve about 10 mg in 25 mL of water, and to this solution add 1 mL of a solution prepared by mixing 20 mL of copper sulfate solution (1 in 5) and 8 mL of pyridine: a precipitate is formed in the blue solution within 1 minute.
Water: 0.5%.
Residue: not more than 0.1%.
Heavy metals: 0.001%.
Limit of maleic acid: To pass the test. Not more than 0.1% of maleic acid is found.
Organic volatile impurities: meets the requirements.
Specifications of Fumaric Acid FCC Food Grade
(E)-Butenedioic Acid; trans-1,2-Ethylenedicarboxylic Acid
C4H4O4 Formula weight 116.07
INS: 297 CAS 110-17-8
FEMA: 2488
DESCRIPTION
Fumaric Acid occurs as white granules or as a crystalline powder. A 1:30 aqueous solution has a pH of 2.0 to 2.5. It is soluble in alcohol, slightly soluble in water and in ether, and very slightly soluble in chloroform.
Function: Acidifier; flavoring agent.
REQUIREMENTS
Identification: The infrared absorption spectrum.
Assay: Not less than 99.5% and not more than 100.5% of C4H4O4, calculated on the anhydrous basis.
Lead: Not more than 2 mg/kg.
Maleic Acid: Not more than 0.1%.
Residue on Ignition: Not more than 0.1%.
Water: Not more than 0.5%.
Please visit Hazard Statement of Fumaric Acid USP NF FCC Food Grade Manufacturers.
Ferrous Fumarate
CAS Number: 141-01-5, USP BP Ph Eur IP FCC Food Grade Suppliers Exporters, Manufacturers
Please visit Safety Data Sheet of Ferrous Fumarate Manufacturers.
Specifications of Ferrous Fumarate BP Grade Ph Eur
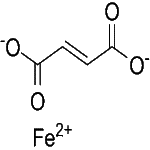
C4H2FeO4 --- 169.9 --- CAS 141-01-5
DEFINITION
Iron (II) (E)-butenedioate.
Content: 93.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance: Fine, reddish-orange or reddish-brown powder.
Solubility: Slightly soluble in water, very slightly soluble in ethanol (96 per cent).
IDENTIFICATION
A. Thin-layer chromatography.
B. Mix 0.5 g with 1 g of resorcinol R. To 0.5 g of the mixture in a crucible add 0.15 ml of sulphuric acid R and heat gently. A dark red semi-solid mass is formed. Add the mass, with care, to 100 ml of water R. An orange-yellow colour develops and the solution shows no fluorescence.
C. The filtrate obtained during preparation of the test solution in identification test A gives reaction (a) of iron (2.3.1).
TESTS
Solution S: Dissolve 2.0 g in a mixture of 10 ml of lead-free hydrochloric acid R and 80 ml of water R, heating slightly if necessary. Allow to cool, filter if necessary and dilute to 100 ml with water R.
Sulphates: Maximum 0.2 per cent.
Arsenic: Maximum 5 ppm.
Ferric ion: Maximum 2.0 per cent.
Cadmium: Maximum 10.0 ppm.
Chromium: Maximum 2.00 × 102 ppm.
Lead: Maximum 20.0 ppm.
Mercury: Maximum 1.0 ppm.
Nickel: Maximum 2.00 × 102 ppm.
Zinc: Maximum 5.00 × 100 ppm.
Loss on drying: Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105C.
Specifications of Ferrous Fumarate USP Grade
C4H2FeO4 --- 169.90
2-Butenedioic acid, (E)-, iron(2+) salt.
Iron(2+) fumarate CAS 141-01-5
Ferrous Fumarate contains not less than 97.0 percent and not more than 101.0 percent of C4H2FeO4, calculated on the dried basis.
Identification:
A: To 1.5 g add 25 mL of dilute hydrochloric acid (1 in 2). Dilute with water to 50 mL, heat to dissolve, then cool, filter on a fine-porosity, sintered-glass crucible, wash the precipitate with dilute hydrochloric acid (3 in 100), saving the filtrate for Identification test B, and dry the precipitate at 105C: the IR absorption of a potassium bromide dispersion of the dried precipitate so obtained exhibits maxima only at the same wavelengths as that of a similar preparation of USP Fumaric Acid RS.
B: A portion of the filtrate obtained in the preceding test responds to the tests for Iron.
Loss on drying: Dry it at 105C for 16 hours: it loses not more than 1.5% of its weight.
Sulfate: Transfer 1.0 g to a 250-mL beaker, add 100 mL of water, and heat on a steam bath, adding hydrochloric acid drop wise, until complete solution is effected (about 2 mL of the acid will be required). Filter the solution if necessary, and dilute the filtrate with water to 100 mL. Heat the filtrate to boiling, add 10 mL of barium chloride TS, warm on a steam bath for 2 hours, cover, and allow to stand for 16 hours. (If crystals of ferrous fumarate form, warm the solution on the steam bath to dissolve them.) Pass the solution through ash less filter paper, wash the residue with hot water until, with the addition of ammonium sulfide TS, a black precipitate is no longer formed in the filtrate, and transfer the paper containing the residue to a tared crucible. Char the paper, without burning, and ignite the crucible and its contents at 600 to constant weight: each mg of residue is equivalent to 0.412 mg of SO4. Not more than 0.2% is found.
Arsenic: Transfer 2.0 g to a beaker, and add 10 mL of water and 10 mL of sulfuric acid. Warm to precipitate the fumaric acid completely, cool, add 30 mL of water, and filter into a 100-
mL volumetric flask. Wash the precipitate with water, adding the washings to the flask, add water to volume, and mix. Transfer 50.0 mL of this solution into the arsine generator flask, and dilute with water to 55 mL: the resulting solution meets the requirements of the test, the addition of 20 mL of 7 N sulfuric acid specified for Procedure being omitted. The limit is 3 ppm.
Limit of ferric iron: Transfer 2.0 g, accurately weighed, to a glass-stoppered, 250-mL conical flask, add 25 mL of water and 4 mL of hydrochloric acid, and heat on a hot plate until solution is complete. Insert the stopper in the flask, and cool to room temperature. Add 3 g of potassium iodide, insert the stopper in the flask, swirl to mix, and allow to stand in the dark for 5 minutes. Remove the stopper, add 75 mL of water, and titrate with 0.1 N sodium thiosulfate VS, adding 3 mL of starch TS as the end-point is approached. Not more than 7.16 mL of 0.1 N sodium thiosulfate is consumed (2.0%).
Limit of lead: To pass the atomic absorption spectrophotometer test. The Test solution does not exceed that of the Standard solution (0.001%).
Mercury: To pass the test of 3 µg per g.
Ferrous Fumarate IP Grade
Specifications of Ferrous Fumarate FCC Food Grade
Iron (II) Fumarate FCC
C4H2FeO4 --- Formula weight 169.90 --- CAS 141-01-5
DESCRIPTION
Ferrous Fumarate occurs as a red-orange to red-brown powder. It may contain soft lumps that produce a yellow streak when crushed. It is soluble in water and in alcohol.
Function: Nutrient.
REQUIREMENTS
Identification:
A. Add 25 mL of 1:2 hydrochloric acid to about 1.5 g of sample, and dilute to 50 mL with water. Heat to effect complete solution; then cool; filter on a fine-porosity, sintered glass crucible; wash the precipitate with 2:100 hydrochloric acid, saving the filtrate for Identification Test B; and dry the precipitate at 105C. Add 3 mL of water and 7 mL of 1 N sodium hydroxide to 400 mg of the dried precipitate, and stir until solution is complete. Add, drop wise, 2.7 N hydrochloric acid until the solution is just acid to litmus; add 1 g of pnitrobenzyl
bromide and 10 mL of alcohol; and reflux the mixture for 2 h. Cool, filter, and wash the precipitate with two small portions of a 2:1 alcohol:water mixture, followed by two small portions of water. The precipitate, recrystallized from hot alcohol and dried at 105°C, melts at about 152C.
B. A portion of the filtrate obtained in Identification Test A gives positive tests for Iron.
Assay: Not less than 97.0% and not more than 101.0% of C4H2FeO4, calculated on the dried basis.
Ferric Iron: Not more than 2.0%.
Lead: Not more than 2 mg/kg.
Loss on Drying: Not more than 1.5%.
Mercury: Not more than 3 mg/kg.
Sulfate: Not more than 0.2%.
Please visit Hazard Statement of Ferrous Fumarate USP BP Ph Eur IP FCC Food Grade Manufacturers.
Fumaric Acid USP NF FCC Food Grade CAS Number 110-17-8 & Ferrous Fumarate USP BP Ph Eur IP FCC Food Grade CAS Number 141-01-5 Supplier Exporter, Manufacturer:
Annie Chemie P Ltd
Mumbai 4000010, INDIA
With Agents and offices in UAE, USA, Europe.
e-mail: info@anniechemie.com
Copyright and Usual Disclaimer is Applicable.
May 31, 2025
Exporters to USA, Canada, China, Europe, UAE, Nigeria, Algeria, Turkey, Mexico, Brazil, Chile, Argentina, Australia, Dubai etc.
Perfection is made up of small things and that is a big thing.
